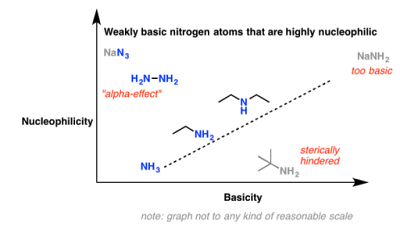
A particular advantage of solid-state NMR and NMR
spectroscopy, in general, is its ability to provide information on the dynamics
of biomolecules.
Pros:
In solid-state NMR spectroscopy, motions on a
broader timescale (from the nano- to the millisecond timescale and beyond) can
be detected. For example, the nano- to millisecond timescale can readily be
explored by measuring the spin–lattice relaxation in the laboratory (R1) or the
rotating frame (R1r), while real-time solid-state NMR allows the course of
protein refolding or of enzymatic reactions to be followed.
There is no protein size limit in solid-state NMR
spectroscopy.
In liquid state, the anisotropic interactions give
rise to fluctuating local fields which themselves manifest as relaxation effect
in the spectra. In contrast, the rapid tumbling of molecules is absent and the
anisotropic interactions give rise to a distribution of resonances reflecting
the distribution of molecular orientations within the sample. These
distributions, on analysis, can provide wealth of information: the anisotropic interactions experienced by
the nuclear spin, the local electron distribution around a nucleus, and relative proximity between individual spins.
Using the structural and dynamic information encoded in the interactions experienced by the nuclear spin, it is possible to determine how the structure and dynamics present within membrane proteins relates to their function, and how these molecular species interact within the membrane.
Using the structural and dynamic information encoded in the interactions experienced by the nuclear spin, it is possible to determine how the structure and dynamics present within membrane proteins relates to their function, and how these molecular species interact within the membrane.
Cons:
In solution state, resolution is provided mostly by
nature because the anisotropic spin interactions that can broaden NMR lines are
motionally averaged. In the solid state, motional averaging is less efficient
because of reduced mobility, and considerably broadened spectra are acquired in
this case. Line narrowing, therefore, must be performed artificially:
manipulation of the Hamiltonian to dissect the anisotropic interactions or to
suppress their influence on NMR spectra in a controlled manner.
With regards to the protein size, technical
challenges remain to be solved before dynamics can be routinely measured at
atomic resolution in large proteins.
Additionally, with respect to solution state
NMR integral membrane proteins embedded in detergent micelles are still
challenging.